|
4:00 P.M.
October 4, 2007
Power to Go!

Franklin D. Peele
Assembly Room
A. K. Smiley Public Library
Synopsis
The ever-increasing array of electrical and electronic devices available to assist, inform and entertain us means that more and more we need portable electric power. The typical modern home has a growing range of devices that are either battery powered or use batteries as backup power. This paper takes a look at historical batteries, those we have available in a bewildering variety today, and batteries coming in the near future. It examines the early work of Volta, Planté and others in wet cells, more modern wet and dry cells and their developers, the advantages and drawbacks of the different types, and discusses emerging battery technologies for portable and transportation-based applications.
Preface
For my last Fortnightly paper I chose the subject of electric lighting, with an emphasis on emerging technologies. This was a natural outgrowth of my abiding interest in, and use of, light as a photographer. The topic of today’s paper is somewhat related to that last one, and I hope that it will hold some interest to those of you who take the occasional photograph and to those of you who don’t as well. All of us are affected more and more these days by the need for portable electric power in an ever-increasing array of devices we use to assist, inform and entertain us. Today we’ll look at portable electric power or, more simply put, the battery.
Power to Go!
There’s an old joke that describes a flashlight as “a metal tube used for storing dead batteries”. If we were to bring that one up to date, we might want to expand a bit on its options. Nowadays, the typical home has quite a range of devices that are either battery powered or use batteries as backup power. There’s everything from a stunning variety of flashlights to portable entertainment systems (don’t today’s teenagers seem to come with wires leading to their ears from an i-Pod as “standard equipment”?) and cell phones, personal digital assistants, remote controls for everything from the TV to the garage door, digital cameras, portable tools, kitchen appliances and radio-controlled toys, and let’s not forget more serious inventions like heart pacemakers and powered wheelchairs. In a quick survey of my own home, I counted 21 power tools, an even dozen appliances, 20 radios and other entertainment devices, 8 telephones, 41 cameras and accessories, 33 clocks, watches and weather instruments, 6 car key remotes, 28 assorted other remote controls, 9 computers and related devices, 14 flashlights and a few others. The surprising total? 195 gadgets using batteries!
What makes all these devices practical, of course, in one form or another, is the battery. We’re going to take a good look at batteries to appreciate where they’ve come from, what we have available today and to get a peek at what’s coming in the near future. Although the time we have available won’t allow us to cover all of the interesting territory, meaning we’ll have to leave out interesting topics like fuel cells and solar energy, I promise that what we do address should leave us all “charged up”!
First, a little history
Ironically, batteries may precede modern knowledge of electricity.

|
Figure 1: “Prehistoric” battery (?)
Clay jar with iron rod surrounded by copper cylinder. When filled with vinegar as an electrolyte, it produces 1.1 volts DC.
|
Terra cotta vessels unearthed in what is now Iraq are believed by some to have been used as primitive batteries by the Parthians, who ruled Baghdad from as early as 250 BC, or their successors the Sassanids from the same area as late as 640 AD . When such a clay jar is filled with grape or lemon juice or vinegar as an electrolyte, its copper and iron electrodes can carry small currents adequate to electroplate metals or provide mild shocks which might have been used in religious ceremonies or for medicinal purposes. There’s been much debate and controversy surrounding these clay pots since their discovery in 1936, but it’s at least intriguing to speculate that battery power may have been put to some use even before man understood much about the fundamentals of electricity.
When our Historian Dr. Dick Moersch related earlier in today’s meeting, our 1749 th, about the significant happenings of the year 1749, he didn’t have much to say about developments in electric power or related inventions because things were pretty quiet on that front. He specifically didn’t mention the inventive exploits of electrical pioneer Allesandro Volta, but then in 1749 he was only a lad of 4. Indeed, the inventor of the first verifiable battery didn’t come up with his “voltaic pile” until 1800.
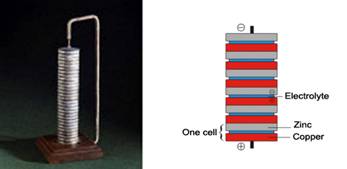
Figure 2: Volta’s Voltaic Pile Battery, 1800
This first battery had serious shortcomings – low capacity, short life, leakage of messy chemicals among them – but once Volta kicked things off, improvements came from many quarters. There were numerous combinations of metal electrodes and liquid electrolytes, but all had one thing in common. To generate a flow of electrons they depended on electrochemical reactions which in one way or another depleted the very materials of which they were made. That is, each would be permanently exhausted when its chemical reactions were spent.
Then in 1859, French physicist Gaston Planté invented the lead-acid battery, the first that could be recharged by passing a reversed electric current through it.

Figure 3: Planté’s Rechargeable Lead-Acid Battery, 1859
Planté’s cell consisted of lead plates wound in a spiral as electrodes, submerged in a sulfuric acid electrolyte. He and others improved on his design by forming the lead plates into grids, a design still in common automotive use in lead-acid batteries today, but as we’ll see later, Planté really was onto something with his spiral idea.
Let’s pause just now and settle an issue that might be bothering some of you. In several instances I’ve been using the term “battery” to describe what are technically “cells”, as if the two terms were interchangeable, and of course they’re not. Strictly speaking a cell (as in the example of Figure 3 ) is a combination of a cathode or positive electrode, an anode or negative electrode, and an electrolyte in a container. A battery is two or more cells connected together to increase the voltage or current available. So if we were today observing the strictest scientific rigors, I wouldn’t point to an Energizer “D” cell and call it a flashlight “battery”. But both my desk dictionary, Merriam-Webster’s New Collegiate, 1977, and The Random House Dictionary of the English Language, Unabridged , 1996, allow both usages. So I don’t feel restricted by formality, and will intermix the terms “cell” and “battery” at will.
The brilliance of Planté’s battery was its rechargeability. Instead of having to replace an exhausted cell after a short period of use as with all previous inventions, Planté was able to feed a current back into the unit and thus rejuvenate it for additional service. Today we call such rechargeables secondary batteries, and those that are not rechargeable are called primaries. The starting battery in your car is probably the best-known example of a secondary battery, and the common flashlight battery (e.g. the “Energizer” or “Duracell”) is usually a primary type.
Planté was just one of many innovators working to improve early batteries. Along with the Englishmen Cruickshank, Sturgeon and Daniell there were the Welshman Grove, as well as Planté’s fellow countrymen Fauré, Callaud and Leclanché, to name a few. Improvements there were, but until 1887, all of these devices shared a major drawback – their wet electrolytes.
Imagine the inconvenience of handling one or more containers of liquid if you wanted your battery-powered device to be portable. Typically the liquid was sulfuric acid, which added a bit to the challenge of moving it around. What was needed for portability was a dry battery, and that breakthrough occurred in 1887. Frederik Hellesen in Denmark and, working independently, Carl Gassner in Germany, developed successful paste electrolytes in closed metal shells, creating batteries that could operate in any orientation without spilling. It’s not clear today whether Hellesen or Gassner was actually first to succeed, but in the U.S. the National Carbon Company improved on Gassner’s design and, in 1896, marketed the Columbia dry cell.
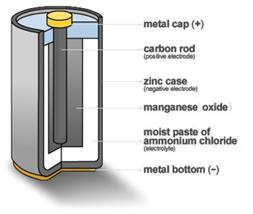
Figure 4: Carbon-Zinc Dry Cell
This was the first mass-produced dry battery, and it sparked the invention of the flashlight in the same year. The basic 1896 National Carbon design, known as the carbon-zinc cell, is still being manufactured today.
Modern Dry Batteries
Now that we’ve forged ahead to more modern times, let’s discuss several dry battery types and their utilization. We’ll break them down into primary, or disposable, and secondary, or rechargeable, varieties; let’s begin with the primaries.
Primary (Non-Rechargeable) Dry Batteries
While the venerable carbon-zinc cell is still around and low-priced, its technology has been bettered by more modern developments. Most popular among these is the alkaline cell, first marketed in 1959; its chemistry provides considerably heavier-duty service than the carbon-zinc cell. Heavily advertised examples are the Energizer and the Duracell, whose commercials harken all-too-well to the old Madison Avenue cliché of “sell the sizzle, not the steak”. One could quite logically conclude that the intensity of their ad blitz is inversely proportional to the differences between these two products. Indeed, it’s enlightening to look at the November, 2007, issue of Consumer Reports magazine and realize that, among tested alkaline batteries in the popular AA size, performance is essentially equal across the board while price per battery runs the gamut from $1.50 for a Duracell “Ultra Digital” to 21 cents for Costco’s house brand.
Getting past the branding issues, alkalines are good choices for devices that draw low-to-moderate current over sustained periods, such as flashlights, toys, radios, CD players, clocks and remote controls. Alkalines usually have a shelf life of several years if properly stored, and many are stamped with an expiration date for handy reference. They come in all the common sizes – AAA, AA, C, D, and 9v – and in many specialty types.
A newer technology, nickel oxyhydroxide, costs about the same as alkalines. These are proving to be a better choice in high-current devices like digital cameras, but they aren’t as good a choice as alkalines for lower-current applications. Nickel oxyhydroxide brand names include Duracell “Power Pix” and Panasonic “Digital Extreme”
For sale since the 1970’s, lithium primary cells capitalize on the fact that lithium is the metal with the lowest density and the greatest electrochemical potential and energy-to-weight ratio . Lithium’s promise for battery use was actually first explored in 1912, and research continues briskly today as we’ll see when we explore modern rechargeable batteries a bit later. As to lithium primary cells, they cost more than either the alkaline or nickel oxyhydroxide types, but in high-current applications, their performance more than justifies the extra cost. Consumer Reports tested Energizer’s “e2 Lithium” AA cells and found that, while costing about one-and-a-half times as much as alkaline batteries of the same size, they can take up to six times more digital flash photos per set.. Because of their very long lives, lithium primary batteries are especially recommended for household smoke alarms.
While the common battery sizes mentioned above represent the lion’s share of the disposable market, there’s another segment that bears mentioning here – so-called “button cell” batteries.

Figure 5: Button Cell
Also called “coin cells”, these are commonly found in watches, hearing aids and other small devices with modest power demands. They’re available in a variety of electrochemical types, including alkaline, lithium, silver oxide and zinc-air. Once in widespread use was the mercury cell, which was specified by manufacturers for millions of cameras, exposure meters and similar devices in the 1960’s, 70’s and 80’s. When mercury was identified as an environmental hazard, mercury batteries became unavailable in the U. S. almost overnight. What had made mercury cells so popular with equipment makers was their discharge characteristic: they supplied an unwavering 1.35v DC from when first installed to very near their exhaustion, meaning the devices in which they were used did not need voltage regulating circuitry. This cut production costs and equipment bulk, so to the manufacturers, mercury cells were clear winners. When they were banned, the remaining available battery types lacked the mercury cells’ voltage constancy, so manufacturers had to redesign equipment to include regulating circuits. In many cases, this coincided with the introduction of more sophisticated – even computerized – cameras, so adding the additional circuitry did not cause major upheaval. The bigger challenges were for photographers and other users who often had to buy new equipment, because no suitable replacement battery could be found for their older gear. Zinc-air cells were developed as replacements for some mercury cells, but in many cases, if the output of the battery had to be precisely 1.35 volts, the equipment wound up being scrapped.
A significant drawback of button cells is their higher cost per unit of energy, so their best use is in applications where larger batteries would be impractical.
Secondary (Rechargeable) Dry Batteries
While we’ve seen that comparison shopping among dry cells can yield relatively good buys, that’s not the complete story. Economically, when compared to the best of rechargeable batteries, disposables just don’t pencil out. A rechargeable AA cell, for example, may cost four times as much as its non-rechargeable cousin, but be reusable hundreds of times. The trick is to choose the right types of rechargeable batteries and chargers, because there is none that’s “best” for all purposes. Here’s a rundown of commonly-available rechargeables:
Nickel-Cadmium, commonly referred to by its chemical abbreviation NiCd, pronounced Ny-Cad. Invented by Waldmar Jungner in Sweden in 1899, NiCds became popular in the 1960’s for their many advantages:
Nickel-Cadmium Rechargeable Batteries
Advantages
• Moderate power per unit of mass (150 W per kg)
• Able to withstand high charge and discharge currents
• Relatively inexpensive
Disadvantages
• Nominal cell voltage 1.25v compared to alkaline cells’ 1.5v
• “Memory” effect if not fully charged and discharged regularly
• Self-discharge rate of 10% per month
• Environmental hazards of discarded cadmium
Figure 6: Advantages and Disadvantages of NiCd Batteries
NiCds have sold by the tens of millions, but are rapidly falling out of favor as newer technologies emerge. More modern batteries have gained in energy efficiency over the NiCd and don’t have the environmental baggage posed by cadmium. The first of these we’ll discuss is the Nickel Metal Hydride.
The Nickel Metal Hydride cell (abbreviated NiMH) is a variant of the NiCd which replaces the latter’s cadmium electrode with a mixture of rare earth alloys such as lanthanum, cerium and neodymium. It was invented by the Lithuanian-American Stanford Ovshinsky in the 1980’s. Ovshinsky, whose Michigan firm Energy Conversion Devices, Inc. has been a significant player in the development of alternate energy for transportation and in solar energy research, would himself make a fascinating subject for a Fortnightly paper. His 350 or so patents put him in a league populated by few other innovators; Thomas Edison’s name comes quickly to my mind as a parallel. Among the alliances he and his company have formed are ones with Toyota, for development of the batteries used in the Prius hybrid cars, and General Motors, with whom he worked on the EV-1 electric car program.
Summarized here are the main pros and cons of the NiMH battery:
Nickel-Metal Hydride Rechargeable Batteries
Advantages
• High power per unit of mass (up to 1000 W per kg)
• Minor if any “memory effect” (reversible through charging)
• Able to withstand moderate to high discharge currents
• Disposable without special environmental concerns
Disadvantages
• Nominal cell voltage 1.2v compared to alkaline cells’ 1.5v
• Best results obtained with “smart” chargers
• Self-discharge rate of up to 30% per month in older designs
Look for new “Precharged” cells with low self-discharge rate
Figure 7: Advantages and Disadvantages of NiMH Batteries
The Lithium-ion (“Li-ion”) battery has become a workhorse in portable electronic devices such as cell phones and laptop computers. Like the lithium primary cell with which it shares a number of similarities, lithium-ion secondary cells offer excellent energy-to-weight ratios. Unfortunately, there have been a number of high-profile recalls of these batteries due to high-temperature operation – even fires – resulting in damage to computers and nearby equipment. Much is being done to continue development of lithium-ion batteries. In the meantime Lithium-polymer (“LiPo”) technology, a refinement of the principle, shows great promise and is the chosen power for Apple’s iPhone and some other leading-edge applications. Here’s a summary of lithium secondary batteries’ pros and cons:
Lithium-ion and Lithium-polymer Rechargeable Batteries
Advantages
• Very high power per unit of mass (up to 2800 W per kg)
• No “memory effect”
• Self-discharge rate of only 5% per month
• Can be shaped to conform to equipment contours
Disadvantages
• Best utilized in specially-designed applications
• Best results obtained with “smart” chargers
• Contamination in manufacture may lead to self-ignition
Figure 8: Advantages and Disadvantages of Li-ion and LiPo Batteries
We’ve discussed several types of primary and secondary dry batteries. Modern wet cells have also come a long way, and remain important in the rechargeable battery market – especially in heavy-duty applications where weight is not so much a factor, like starting the internal-combustion engines of our cars and trucks. Lead-acid cells, which you’ll recall were invented in 1859 by Gaston Planté, are still the battery technology under the hoods of most of our vehicles. They are not, however, without improvements.

Figure 9: Lead-acid Automotive Battery
Today’s Starting or Cranking Battery is optimized to deliver relatively brief bursts of high current to start an engine and then shortly thereafter be recharged by the engine’s alternator. As long as this type of battery is not discharged too deeply and is charged properly, it will typically give good service for several years before needing replacement. Looking very similar on the outside but having different internal construction to withstand the rigors of deep discharge and recharge cycling, the Marine or Deep Cycle Battery is better suited for service in recreational vehicles, golf carts, boats and other applications where there may be relatively long periods of discharge between charges. If starting batteries were subjected to regular deep cycles, most of them would suffer early failure. On the other hand, deep cycle batteries are not intended to deliver very high current on a regular basis. As in so many things, it’s important to select the right battery for the task at hand!
I mentioned earlier that Gaston Planté’s breakthrough original lead-acid cell design was a spiral configuration (Feel free to refer back to Figure 3) and that he and others had improved on his invention with grid-based designs. Here’s an illustration of such a grid and how it’s used in the construction of a typical lead-acid automotive battery today:
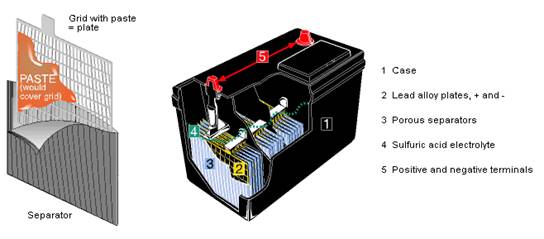
Figure 10: Lead-acid Automotive Battery Interior
The grid is made of lead, typically alloyed with antimony to add strength. The grid’s holes are filled with a paste of red lead, bolstered with compounds which vary from one manufacturer to another, to enhance performance. Each cell develops a nominal 2v DC, so a 12v battery has 6 cells usually arrayed in a straight-line configuration like the battery in Figure 10.
As anyone who has ever lifted one knows, lead acid batteries are quite heavy. Whether that’s an advantage or disadvantage depends on the application: in an electric forklift, for example, the batteries provide both motive power and counterweights to the lifted load. When compared to the latest dry rechargeables, however, the lead acid wet cell won’t win any contests of power per unit of mass.
There are inherent weaknesses in the grid plate design found in the typical vehicle battery. For one thing, it doesn’t handle vibration as gracefully as one would wish. Even more seriously, the very process of charging and discharging creates ever-changing dimensions of the paste composition pushed into the grid. Over time this weakens the structure and parts of it are actually shed, falling into the bottom of the battery case and reducing performance. One attempt to forestall this deterioration is the absorbed glass mat, or AGM, battery design in which the grids are separated by fiberglass mats which have absorbed the electrolyte, so there’s no longer a spillable liquid in the battery case. Equally important, the fiberglass mats actually press against the plates so, no longer needing additive alloys for strength, they can be purer lead, enhancing their electrical properties. The result is a lead-acid battery that’s stronger, lighter, longer-lasting, and can be operated in any orientation without fear of electrolyte leakage (think of an aircraft performing aerobatic maneuvers). A somewhat similar approach is the “gel-cell”, in which the electrolyte is gelled to eliminate leakage. Both of these designs can be referred to as “sealed lead acid” batteries, although each does have valves to relieve excess gas pressure which could build up in the event of over-charging.
Before we leave the lead acid wet cell, let’s revisit Planté’s original plate design and find it reborn in a very modern adaptation. Imagine the absorbed glass mat battery described above, but with the plates and separator mat wound together in a tight spiral. In doing so we’ve greatly strengthened the physical integrity of the cell, while concurrently reducing its size. The resulting spiral-cell design has the potential of outperforming and outlasting traditional grid plate battery designs, and that’s turning out to be the case for many users who have adopted the somewhat oddball looking batteries made this way.

Figure 11: Spiral-Cell AGM Battery
I correspond with the retired owner of an auto repair shop in Denver who has two Optima brand spiral-cell batteries he’s been using for 17 years; he insists he completely ignores them and they just keep on cranking every time they’re needed. That’s decidedly not typical service, but I know of a number of other owners who report considerably longer life from their Optima batteries than they were getting with conventional batteries in the same vehicles. My personal conclusion is that, although they cost more initially, they’re well worth considering for a vehicle one plans to keep for a while. The one you see here today is waiting in the wings to go in my next car that needs a battery.
With the lead-acid wet cell having been around almost 150 years now, even with the improvements noted above, surely there must be newer technologies that have the potential to replace lead-acid as king-of-the-hill in transportation batteries. Indeed, if nothing else we can thank high petroleum prices for research going on in laboratories around the globe to find the breakthrough battery that could power the car of tomorrow. Hybrid gasoline-electric vehicles have been important steps in that direction, and have spurred significant innovations in battery design. Porsche, for example, is readying a hybrid version of their Cayenne sport-utility vehicle for market. Their test cars are currently equipped with nickel metal-hydride batteries, but Porsche is holding out for the latest lithium-polymer batteries which promise to deliver up to twice the energy from half the weight.
However, even if we all somehow switched to Toyota Priuses or other hybrid vehicles next week we’d still be dependent on gasoline. When you look at the driving patterns of many city-dwellers, the all-electric car is an appealing way to ease appalling fuel bills. Even using presently-available batteries, a car like General Motors’ ill-fated EV-1 with its 160 mile range would meet the daily local needs of many of us. The dream of doing a day’s driving without ever thinking of what gas stations are charging, then plugging the car in for the night to “refuel” at an equivalent cost that works out to about 200 miles per gallon, is very intriguing. Standing in the way of that dream is the industry that could make it happen, with its billions of dollars of investment in the traditional ways of building automobiles and the infrastructure to fill them with petroleum-derived fuels. In that regard, the Japanese and European manufacturers are generally well ahead of their American counterparts. It’s interesting that while the Detroit Big Three spent years and millions of dollars lobbying congress to convince them that it was not feasible to dramatically increase vehicle fuel efficiency (so laws to require such mileage increases would be wrong-headed), Japanese and European automakers were spending millions of dollars on research – and they made their vehicles dramatically more fuel efficient.
Here’s one take on the near-term future of battery development for transportation, according to the journal of the Institute of Electrical and Electronic Engineers:
The future of battery electric vehicles depends primarily upon the cost and availability of batteries with high energy densities, power density, and long life, as all other aspects such as motors, motor controllers, and chargers are fairly mature and cost-competitive with internal combustion engine components. Li-ion, Li-poly and zinc-air batteries have demonstrated energy densities high enough to deliver range and recharge times comparable to conventional vehicles…
The cathodes of early 2007 lithium-ion batteries are made from lithium-cobalt metal oxide. This material is pricey, and can release oxygen if its cell is overcharged. If the cobalt is replaced with iron phosphates, the cells will not burn or release oxygen under any charge. The price premium for early 2007 hybrids is about US $5000, some $3000 of which is for their NiMH battery packs. At early 2007 gasoline and electricity prices, that would break even after six to ten years of operation. The hybrid premium could fall to $2000 in five years, with $1200 or more of that being cost of lithium-ion batteries, providing a three-year payback.
Voelcker, J. "Lithium Batteries for Hybrid Cars"IEEE Spectrum (January 2007)
There’s no doubt about it, we live in electrifying times. Batteries are good and getting dramatically better. They promise to have ever-greater influence in our lives, solving problems and making possible technological wonders that were unimaginable to the pioneers whose earlier work made them feasible. Just a word of caution – when you go home and hug a battery today, be safe and don’t create any short circuits!
© Franklin D. Peele 2007
Acknowledgements
While much of the information in this paper comes from my own experiences as a user of batteries in over fifty years as a photographer, electronic hobbyist and car enthusiast, I am much indebted to the Internet as a multi-faceted source of material. Particularly helpful have been Wikipedia, the Free Encyclopedia, and Isidor Buchmann’s batteryuniversity.com for historical fact-checking. Due to the relative ease with which material can be placed on Internet sites, however, I have double-checked by using multiple sources whenever possible in extracting information for this paper. The responsibility for any errors of fact is entirely my own.
Biography
Franklin D. Peele was born in North Carolina in 1940. After high school he entered the U. S. Navy, where he rose through the enlisted and warrant officer ranks to earn a regular commission and eventually retired as a Commander. All thirty years of his naval service were in various aspects of photography.
He earned the Bachelor of Arts in Cinema and the Master of Science in Film Education from the University of Southern California.
Since retiring from the Navy in 1988 he and his wife Sue have made their home in Redlands. They have two grown children and four grandchildren. A Certified Professional Photographer specializing in commercial and fine art photography, he is the owner of Pacific Photographic. He also teaches photography courses for the Redlands Adult School and gives private instruction in photographic techniques.
He was admitted to membership in Redlands Fortnightly on April 12, 1990. His local activities have included service on the boards of the Kiwanis Club and Kiwanis Scholarship Foundation, San Bernardino County Museums Foundation, and Friends of Prospect Park. He is a past president of Redlands Fortnightly (in 2001) and of Redlands Meals on Wheels. He is a four-term past president and life member of the Redlands Camera Club, two-term past president and life member of the Inland Empire Professional Photographers and Videographers, and past national president and life member of the National Association of Naval Photography.
His previous Fortnightly papers have addressed the U. S. Navy’s Tailhook scandal, the history of Redlands Meals on Wheels, several photographic topics, and electrical illumination.
Professional affiliations have included the Audio Engineering Society, Information Film Producers of America, Society of Motion Picture and Television Engineers, and Wedding and Portrait Photographers International. He is a member of the Professional Photographers of America and the Professional Photographers of California. He was named Inland Empire Professional Photographer of the Year for 2000 and 2002, and California Scenic Photographer of the Year for 2001.
|