|
4:00 P.M.
December 18, 1980
One Man's Error...........
Scientific Creativity in the History of Biology
by Charles D. Howell Ph.D.
Assembly Room, A. K. Smiley Public
Library
Abstract
The subject is scientific creativity. A
number of scientific discoveries in the area of biology are described. They illustrate
many different modes of success: serendipity and other modes of change, trial and error,
double checking the obvious to find it is not obvious, Correcting of errors impeding
enlightenment, following up of analogous situations, et al.
In all discovery an element of testing is
involved. Often a false conclusion is so obviously right , that it goes unchecked for
generations till a tip-off leads to its testing. Men seeking agents in an expected
situation find a reagent of the opposite nature instead. A conclusion reached that by
analogy should fit other similar settings, on testing falls to fit and may reveal new
conclusions. Often the logical answer fails to work for a crucial bit of the puzzle is in
error or missing. Men may be frustrated till the error is exposed. They may
be awed about the beauty of nature, almost to reverence, only to find it commonplace when
the missing piece falls into place.
Scientific creativity is an indescribably
complex mixture of skepticism, mental preparation, intellectual astuteness, insight,
persistence, luck and dedication to rationality.
Biography of the Author
Charles DeWitt Howell Robertson Professor
of Biology Emeritus, University of Redlands. Curator of Entomology and Invertebrate
Zoology, The San Bernardino County Museum, w.o.p.
Born: Get. 29, 1910, East Bangor, Pa., in
the heart of the slate mining area.
Married: June 8, 1935 to Edith N. Volk, in
the heart of the Berkshires, Mass.
Home: Brooklyn, N.Y. through high school
and college.
Education. Oberlin College, A.B. 1932,
mayor work in zoology, chemistry, and psychology.
The Johns Hopkins University, Ph. D. 1937,
mayor work in physiology,and genetics and cytology.
University of California, Riverside,
1969-70, Post-doctoral research in entomology.
Vocation: Teaching and research in
biological sciences for 45 years in colleges and universities; 25 years at the University
of Redlands.
Publications: Original research in the
fields of physiology, genetics, embryology,and entomology.
Member of Sigma Xi, Honorary Society for
the Advancement of Scientific Research
Fellow of the American Public Health
Service.
Member of American Societies of Zoology,
Physiology and Entomology, and other scientific societies.
Local activities: Leadership training, Boy
Scouts of Amerlca. Past president of the Society of Sigma Xi, Redlands Club. Past
president, Redlands Council of churches. Teacher and Deacon, First Baptist Church and
United Christian Church (Congregational) of Highland.
Hobbies: Archaeology, Astronomy, Music,
Hiking, Nature Study.
One Man's Error...........
Scientific Creativity in the History of Biology
by Charles D. Howell
My subject is human
creativity in the biological sciences, disguised behind a nursery rhyme, "One man's
meat is another man's poison". Two men perform the same experiment and one fails and
the other advances science. One man makes an error, and another uses it as a cue to go on
to success. Sometimes accident, sometimes profound analysis explain the difference.
Sometimes the most creative thinkers are hampered by misconceptions of their times which
they never can overcome.
This paper is not creativity in action,
but the pursuit of other minds in their creative endeavors. I have gotten great joy in
this pursuit, following the often tortuous paths of grew minds attempting to bridge the
gap of the unknown, to untangle the spider web of 1nformat10n and to weave it into a
useful cord of knowledge.
I owe this pleasure to four men whom I
regard as great teachers, who thrilled me with their exact understanding and their
appreciation of our debt to the past. Two were at Oberlin College: Robert Allyn Budington,
gentle and artist Professor of Zoology; and Lawrence A. Cole, mind-boggling and
stimulating Professor of Psychology. Two were at The Johns Hopkins University: Herbert
Spencer Jennings, eminent geneticist and sensitive and kindly teacher; and Phillip Bard,
neurophysiologist, and master showman and lecturer. With them began my adventures in the
discovery of human creativity in the sciences.
My first account comes from the golden age
of American medicine, for the courage and ingenuity of American pioneers always delights
me. About one hundred years ago Dr. W1111am Mayo did one of the first successful
gallbladder operations on a farm near Rochester, Minnesota. He gained the courage to
perform this feat by his fa111 in reasoning from anatomical analogies.
Dr. Mayo earned his living as much as a
veterinarian as by being a physician, especially in the earlier days of his career. His
intense curiosity made him ask his neighbors to let him dissect every farm animal that
died. He sought to learn from every dissection. He discovered that horses, and some other
mammals, have no gall bladders. Since Mammals are all much alike, why couldn't a human
being also survive minus a gallbladder?
There were practical reasons for his
speculations, far he had applied his thinking to relating symptoms of patients to
discoveries he had made on human autopsies. He was sure he could diagnose gallstone pains
frog other pains, in spite of not having and x-ray machine to verify them. He had a
patient who was suffering such pains, and was at the point of preferring death to
continued suffering. Dr. Mayo talked over his theory about the non-essentiality of the
human gallbladder with her, and she agreed to let him operate on her, and to let
Providence determine her fate and that of the doctor's.
Her ample kitchen was converted to an
operating room, and her kitchen table into an operating table. The country surgeon, far
away from the centers of medicine In London and Paris, removed a gallbladder full of
gallstones, and his patient survived to live a painfree life. Repeated precise diagnoses
like this, and successful surgery led to the developing of the famous Rochester Mayo
Clinic.
Studying comparative anatomy I was
thrilled with the successful application of this kind of reasoning. Let me cite another
case of its employment.
Something we almost never see today in
America is a person with a huge disfiguring goitre. Yet, when I was a child I saw many
people in Pennsylvania villages with this affliction, including my own mother. There are
many causes of enlarged thyroid glands, but the common one was a lack of iodine in the
diet due to local soil deficiencies. Once the cause was removed, could the disfiguring
goitres be removed to make the patients look and feel more normal?
To test this, thyroid glands were removed
from some laboratory animals. The thyroid could be removed from a dog without dire
effects, so why not from a human? This was done in various parts of the world about the
same time. In several cases the total thyroid was removed, and the patients succumbed
shortly afterwards with symptoms of tetany. This is a violent uncontrolled contraction of
muscles. The immediate exchange of this information resulted in the rejection of this
operation as a viable surgical procedure.
Physiologists tackled the puzzling
question, why was this operation a failure in humans when it was successful on dogs? An
integration of knowledge from histology, embryology and physiology led to an explanation
of this and to methods of thyroidectomy that would not harm a human patient. The tetany
referred to above was due to calcium deficiency in the blood. The regulation of calcium is
under the control of the parathyroid glands. These glands develops in the human embryo
close to the thyroid, as they do in all mammals. In some species, the parathyroids become
completely imbedded in the thyroid tissue, and in others they are wholly or partially
separate from the thyroid. Unfortunately, man is a species in which they are commonly
imbedded within the thyroid. Today thyroid surgery is done to leave thyroid tissue with
the parathyroids intact so that calcium metabolism is not disturbed by the operation.
So we see that the same kind of reasoning
cannot be used with impunity in two different situation. Supplementary investigation and
testing must be used to support what, without it, lead to erroneous logic.
The pursuit of pure scientific
understanding sometimes leads to unexpected practical discoveries. This happened to Jan
McClean. He was a promising young investigator appointed as an assistant to Dr. W. H.
Howell who was t ying to understand the theory of the clotting of the blood. McClean was a
student in the medical school of The Johns Hopkins University forced to work his way
through medical school, as his parents violently opposed his ambition to get sound
physiological training before pursuing the profession of surgery. This position was a big
jump from some of the less-desirable occupations he had had. Hashed scrubbed decks on
boats in the harbor. He had mined gold in Colorado. He had counted blood cells in an
infirmary, worked in a museum, in a college recorder's office, and finally bn a
position in research. .
His job was to extract a substance from
body tissues of animals that were thought to be factors in the clotting mechanism of
blood. One of these was postulated to be in liver tissue. He was extracting the material
from liver, and was not having the success he'd hoped for. After a trying day he left the
lab without cleaning up. He returned later to the slightly putrid mess of liver extract,
but decided to continue using it-- fresh blood was obtained more easily than fresh liver
extract.
So he applied the old liver material the
fresh blood, set his stop watch and timed the clotting. Well, it didn't clot at first, so
he started over again. It still didn't clot. He took time to make some-fresh liver
extract, and it worked, but if he let it stand for a time, it totally prevented
fresh-blood from clotting. This was not what their experimenting was all about. He
communicated his findings to Dr. E. Howell, and with difficulty got him to witness the
experiment. A new substance seemed to develop in old preparations as the enzymes of the
tissue destroyed the clotting factor. Thus was discovered the important anticoagulant,
heparin.
This is not to condone sloppy laboratory
technique. These men kept track of what they were doing and had a keen eye for the
unexpected. They turned error into success because they had well-prepared minds. But it is
the height of serendipity that while looking for a clotting substance they found an
anti-clotting substance.
No pursuit of truth follows exactly the
path of any other. So do not think that I am describing a model of how to become a
scientist. Each pursuit is a unique adventure of its own. Let us drop back a few centuries
and look at the discovery of some of the classical great principles of biology. The
discovery of oxygen and the discovery that plants produce it in the process of
photosynthesis are considered among the greatest concepts in the history Of science.
However obvious and simple these ideas seem to us today, their origins were beset by many
pitfalls.
One of the potential science laureates of
the eighteenth century was Joseph Priestley, a minister of the Gospel, but not an ordinary
one. He was a British dissenter who supported the American revolution, which gained him
few friends in England. Later on he also supported the French revolution, which earned him
positive animosity. When the Bastille fell, riots burst out in England, and rioters burned
not only his laboratory, but his church and his home. He escaped in disguise, eventually
fleeing to America. He made his home in a village well-known to George Armacost, Carlyle,
Pa., the home of Dickinson College. In the college museum many of Priestley's apparatus
and inventions are on display today.
Priestley was a keen observer, an
ingenious inventor, and a meticulous experimenter. He produced carbon dioxide by pouring
acid over chalk. He went further by dissolving the gas in water, producing a sparkling
water, like Seltzer water of the great spas of Europe. His friends and scientific
colleagues were delighted with this simple discovery and he was awarded the Copley Medal
for this in 1773. He discovered and made laughing gas, which has been a useful anesthetic
down to the present time. He discovered oxygen, a story we shall relate later. He also
discovered that plants revivify air rendered noxious by animal respiration. In addition he
was an- inventor. One of his inventions was the compressed air gun. A model of it was
taken on the Lewis and Clarke expedition, where it was used to mystify Indians, for it
killed a rabbit it was pointed at with neither fire nor smoke nor a visible projectile.
We begin our story in the midst of his
work. At this time the phlogiston theory was accepted to explain certain chemical
reactions. Priestley, quoting an eminent mineralogist of his day,called it "the
greatest discovery that had ever been made in science". It was the first
generalization of chemistry and held sway for almost a century. Priestley, being brought
up on it could not escape the entanglements of its reasoning. Consider the beautiful
transition of a rusty metallic ore into a shiny useful metal. It was like magic, and was
carried out by heating the ore (usually an oxide) in the presence of charcoal. The carbon
of charcoal combined with the oxygen of the ore, reducing it to metal and forming carbon
dioxide. According to the phlogiston theory, the phlogiston of charcoal entered the ore
converting it to pure metal. Such was the magic of phlogiston.
When Nature presents man with riddles, she
often also provides a single exceptional clue for unraveling them. In this case the clue
was cinnabar, the red oxide of mercury. This is the one natural ore that is a pure oxide
without traces of carbonate or sulfates in it. Unlike other ores, it does not require
charcoal for its reduction, and can be reduced with much less heat than usually is
required for reduction. It had always been reduced with charcoal until Bayen, the French
chemist, discovered in 1772 he could reduce it by mere application of heat. However a gas
was produced in the process and was identified erroneously by him as "fixed
air", the eighteenth century name for carbon dioxide. The retesting of this gas was
to fool two other eminent experimenters before its correction was to lead to the discovery
of oxygen.
Priestley repeated Bayen's experiment, and
tested the gas by putting a lighted candle in it. The candle flared up and burned
brightly. This was the kind of reaction he had noted when he put a burning candle into
laughing gas in 1772. Remember that he had no idea of a gas like oxygen doing this. So
naturally he identified the gas as "dephlogisticated nitrous air", his name for
laughing gas. In his previous experience this air suffocated mice, but at this time he did
not put a mouse in it.
 Shortly
after this he went to Paris and communicated this experience to Lavoisier the great French
Chemist, then merely a young man compared to Priestley. Lavoisier promptly repeated the
experiment. He used a test Priestly had invented for the"goodness of air".
According to this test he said the gas evolved was common air, but he noted it was
"purer than common air". When Priestly read this he was puzzled about the
contradiction of his conclusion. Of interest, he later wrote of Lavoisier,"truth has
been a means of leading him into error, error may, in its turn lead him into truth." This was to prove very prophetic. Shortly
after this he went to Paris and communicated this experience to Lavoisier the great French
Chemist, then merely a young man compared to Priestley. Lavoisier promptly repeated the
experiment. He used a test Priestly had invented for the"goodness of air".
According to this test he said the gas evolved was common air, but he noted it was
"purer than common air". When Priestly read this he was puzzled about the
contradiction of his conclusion. Of interest, he later wrote of Lavoisier,"truth has
been a means of leading him into error, error may, in its turn lead him into truth." This was to prove very prophetic.
Let us consider Priestley's test for
goodness of air. It was really a test for oxygen, but that was not known at the time. If
you mix nitrous air (NO or nitric oxide) with half its volume of oxygen it will produce a
red gas (N02 or nitrogen dioxide) which will dissolve fully in water leaving no gas
behind. If used with air, which is four-fifths nitrogen, the nitrogen is left behind.
Priestley had experimented by trial and errand had found that he could get a "maximum
reduction" of air if he mixed one part of nitrous air with two parts of common air.
This mixture,instead of giving three volumes, gave 1.8 volumes. With impure air he got
less of a reduction. Thus the greater the reduction the purer the air.
Since laughing gas is negative to this
test, he did not think of using the test on the new gas. But he soon was led to change his
mind when he found that the new gas did not dissolve in water, as laughing gas does, no
matter how hard or long he shook it. He still had no idea it was common air, but since it
might be like it, he decided at last to perform the "goodness of air" test on
it. It reacted so much like common air that he was about to give in to
Lavoisier's-conclusion. But if it was true, the test must have left behind the suffocating
mephitic air (nitrogen) of common air. To prove this, he inserted a burning candle. The
candle was not snuffed out, but flared up brightly. It was NOT mephitic air'
How strange. Could an animal live in this
air? He inquired if any of his friends had a mouse. Someone did, and Priestley put it in
this sample of gas. The mouse survived twice as long as usual. It was not common air, and
yet a mouse lived in it. He performed the goodness of air test on the sample the mouse had
been in, and it proved to be as good as common air. He repeated the test on this used
sample, and again it was reduced. At last he thought of performing a succession of tests
of the goodness of air on a given sample of the gas.
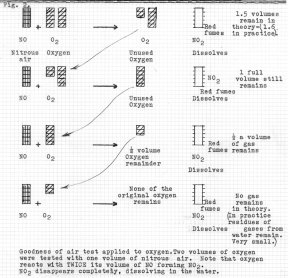 The next
day he started from scratch with a fresh: sample of the gas (see Figure 2) He
reduced it with nitrous air, and found it reduced slightly more than common air. He
repeated this a second time on the once reduced sample, then a third time, and then a
fourth , and not till the fifth attempt was the volume increased by adding nitrous air.It
was four to five times as pure as common air -a new gas. Priestley called in
Dephlogisticated air, but it later came to be known as Oxygen. The next
day he started from scratch with a fresh: sample of the gas (see Figure 2) He
reduced it with nitrous air, and found it reduced slightly more than common air. He
repeated this a second time on the once reduced sample, then a third time, and then a
fourth , and not till the fifth attempt was the volume increased by adding nitrous air.It
was four to five times as pure as common air -a new gas. Priestley called in
Dephlogisticated air, but it later came to be known as Oxygen.
Lavoisier picked up the story from here.
He oxidized mercury with oxygen, and found the oxide had a greater weight than the
original metal. From this he developed the modern concept of chemical reaction, and
established the foundation of modern chemistry. From error he went on to truth as
Priestley prophetically stated.
Prlestley still believed in the phlogiston
theory, but this did not stop him from continuing to make great scientific discoveries.
He was puzzled, and wrote, "The quantity of air which even a small flame requires to
keep it burning is prodigious. It is generally said, that an ordinary candle
consumes...about a gallon a minute. Considering this amazing consumption of air, by fires
of all kinds, volcanoes, etc., it becomes a great object of philosophical inquiry to
ascertain what provision there is in nature for remedying the injury which the
atmosphere receives by this means . " He recognized that without such a
provision,"the whole mass of atmosphere would, in time, become unfit for the purpose
of animal life." Priestley did not rest on his laurels. He was thinking creatively.
He soon discovered that plants purify air,
a tremendous discovery of one of the most beautiful balances in nature. But let us review
briefly some of the reasoning, problems, an pitfalls that beset him.
First, like McClean and Howell,he set out
with an erroneous hypothesis from which he arrived at the correct one. Be reasoned that
since living things, like mice, poisoned the air, then plants should do the same thing. So
he grew plants in his bell jars sealed with water, but he didn't do it in a simple-way.
First,he let a mouse use up the air in a bell Jar, and then he put a plant in it,
expecting it to wilt and die at once- saving time for the experiment. But, the plant did
not wilt and die. After it had thrived for several days, he decided to examine the air by
putting a mouse back in it. To his astonishment, the mouse survived a long time. The air
must have been purified.
Now he reasoned critically. Maybe the air
purifies itself on standing over water. Actually it does, but at such a slow rate that he
wasn't going to be bothered by it. To test this he took some large quantity of air made
noxious by animals living in it till they collapsed. He divided it in two. In one half he
put a plant, and he left the other half standing over water with nothing in it. The plant
thrived. In fact, it seemed to grow even better than in common air. After about a week he
put a mouse in each of these jars. The mouse in the plant side stayed alive instead of
collapsing, but the mouse on the other side collapsed at once. So he felt certain that the
plant, a bit of mint, had rejuvenated the noxious air produced by animal respiration. Be
found that other green plants do this also.
Priestley published these profound new discoveries, and then abandoned his experiments for a number of years. He was very
busy in his pastorate, and could not return to science for five years. When he did, he
tried to sandwich his experiments in a busy schedule. Perhaps he did his work chiefly at
night, and did not put his plants in a lighted room, but at any rate, he failed to be able
to verify his earlier work. The plants did not revivify the air. He had no idea that
sunlight lay behind the successful observations he had made before. He thought he had made
a mistake and was about to deny the validity of his own work.
But other men had already read the work
and had tried to repeat his experiments. One of them saved Priestly the ignominy of saying
he had made an error in his meticulous work. This was Jan Ingenhouez, a Dutch botanist. He
had discovered that plants perform this wonderful function of purling air only when in the
light. So sunlight was brought logo the story, and piece by piece the great Canvas of
scientific understanding had another masterpiece painted in.
Scientific conceptualization does not
progress so much by destroying the past and rendering it obsolete, as by adding to,
mending and patching its past concepts. Let me cite one simple example of the mending of a
great concept, in the present century.
The importance of sugar as a source of
energy developed during the late 19th century. for about seventy years the idea that
muscular work derived its energy from the oxidation of glucose held sway. In 1933 is was
conclusively proven that muscles could perform to their full strength in the total absence
of the:oxidation of glucose. The source of muscle energy Was a newly discovered type of
chemical reaction, the breakdown of an organic triphosphate into a dlphosphate. The loss
of one phosphate released energy to the muscle, not the oxidation of glucose.
This new concept did not utterly destroy
our faith in glucose. It was not faith, but knowledge we had about it. That knowledge was
now revised as we found glucose still held a place of mighty power. It is the source of
the energy that builds up the triph phate. This debunks eating a sugar cube for quick
energy.
So science builds most firmly by addition
and correction. -As a corollary I might add, if you see anyone trying to totally destroy a
great scientific concept, rather than trying to amend or improve it, something may be
wrong in this person's approach.
The unraveling of the mystery of the
chemical-physical nature of the gene also illustrates this gradual process. When I wee in
graduate school the status of proteins wee quite sacred. They make up the warf and woof of
biological matter. We knew of nucleic acids, but they weren't in any beginning
texts. We believed the gene had to be a protein, and if nucleic acids were terribly
prominent in gene material,the gene must be a nucleoprotein.
Did you, as a child, ever fold paper in
many ways and cut off Corners and then unfold the paper to see what kind of beautiful
pattern you had created: Well we did this seriously in graduate school, searching by trial
and error to find a new pattern into which the structures of the parts of nucleoproteins
would fit. We all hoped for a nightmare like Beetle got revealing the structure of the
benzene ring. But no one got such a vision.
One set of facts we had to fit into the
story was the fact, well known in 1930, that the number of molecules of a purine, which I
will call "if' was equal in the gene, to the number of molecules of a pyrimidine I
shall call T''. Also the molecules of "G" another pyrimidine equaled those of
"C", another pyrimidine. This seemed to be a universal law in all genes. Why? or
How7 How could a molecule be made to fit these facts?
We did not have the tools of Xray
diffraction studies, nor know how to measure the angles of chemical bonds, nor the length
or energy of bonds then as could be done by the 50's. But these and more ideas were
available to James D. Watson and Francis Crick in 1953 when they solved the riddle.
Watson was a very young postgraduate
student, 23 years old, when he went to study in the famous Cavendish Laboratory in
England. Of interest to our account is the fact that Cavendish wee one of the last great
Chemists to uphold the Phlogiston Theory, in spite of discovering hydrogen and showing the
molecular structure of water was H2O.
Watson was driven by an almost fanatical
urge to beat the twice Nobel Laureate,Linus Pauling,to the discovery of the chemistry of
the gene, by fair means or foul. He became associated with the brilliant physicist,
Francis Crick, and the two worked hand in hand from there on. They had three-dimensional
metal models made to-scale, of all the parts of the nucleic acid molecule, and tried to
fit them into a coherent scheme.
The irrepressible Watson pried into all
the laboratories where welcome or not, hunting for a hidden clue. One day, while
expounding his vast knowledge of chemistry of purines and pyrimidines, he was politely
interrupted by his host, Jerry Donohue. Jerry was a crystallographer with his feet soundly
on the ground in a field far removed from the biologists pursuit. Elegantly rebuked Watson
for he assurance, and told him textbooks had for years published strictly bogus pictures
of the chemistry of these substances. Their real structure he said, was an alternate form
never shown in textbooks. It was a keto rather than an enol that. should terminate their
molecules.
The embarrassed Watson took it all in, and
went home and began cutting out paper models of keto-ended purines and pyrimidines.
As Crick walked in on him he had just discovered that the new model of "A"
fitted perfectly into "I" in a certain orientation. He at once twisted and
turned and inverted "G" and "C" and found they too could f it
perfectly. In a short time Watson and Crick had substantially conceived of the
double-helix form of DNA. But it took a long time to measure lengths of bond, and angles
of bonds and to make new three-dimensional metal models to verify it.
When it was finished, all parts fitted
with unbelievable precision. Old ideas and new Smashed , and just at a time when Watson
was about to throw out the theory that A=T and G=C. It was true after all, and now they
knew why. They had no doubt that this would earn a Nobel Laureate, and it did nine years
later in 1962.
Pasteur wrote, Chance favors the prepared
minds. The men we have mentioned were not only prepared, they were tenacious and zealous
in their pursuit of ideas. They left no stones unturned to resolve contradictions, to make
reason work. They devised elegant experiments, made ingenious apparatuses, used logic,
trial and error or whatever the human mind could utilize, not being too proud to grasp at
the straws of chance and serendipity. After preparation they had dedication.
Scientists. make errors. But Science has
no dogmatics holding to them. Science can and does correct itself relentlessly as it
advances its understanding of the physical universe. Science exposes the many-sided nature
of creativity. "God created man in his own image" it says in Genesis 2:27. I
have always doubted that this meant that Man looks like God, but I feel man is
nearest God when he is most creative.
Figure 1 shows how Priestley applied
nitrous air to common air. By trial and error he had found he could get the greatest
reduction of volume of common air when mixed with one-half its volume of nitrous air.
Because of the solubility of nitrogen
dioxide in water it practically disappears, its red fumes dissolving in the water.
Instead of three volumes resulting from
his mixture, only 1.8 volumes remains.
Figure 2 shows his application of the same
test to oxygen. One volume of oxygen reacts with two of nitrous air (NO, or nitric oxide).
Only one quarter of the oxygen is used up in the first test, in successive tests it is all
used up by the fourth test. The fifth test would result in a full volumes of NO remaining.
Residual gases escaping out of water into
the chamber, including nitrogen and carbon dioxide, and of course the rare gases xenon and
krypton, left a small volume behind, so the theoretical situation in not quite realized.
|